Biography
Dr. Nan Ma started her academic career at Jilin University in China, where she earned a medical doctoral degree in dentistry. Later, she went on to study tumor immunology at Peking Union Medical College, Chinese Academy of Medical Sciences, getting a master’s degree in immunology. Driven by curiosity, she pursued a Ph.D. in bioengineering with a focus on gene therapy and nanotechnology at the National University of Singapore. After her studies, she gained practical clinical and preclinical experience at the reference and translational center of stem cell therapy, University of Rostock, working closely with the clinical team on stem cells and cardiac regeneration. From 2006 to 2012, she led an independent Helmholtz junior research group exploring stem cell behavior in the cardiovascular system and served as steering committee member of reference and translational center of stem cell therapy (RTC). Dr. Ma's work earned numerous prestigious awards like the Hancock Prize and the Research Award of Mecklenburg-Vorpommern in 2011. Currently, as a professor at Freie Universität Berlin, she leads a research group at the Helmholtz Center Hereon, concentrating on cutting-edge technology for regenerative medicine, specifically in the domains of iPSc-based organoids and biomaterials.
Research interest:
Her research interests lie in studying the interaction between novel biomaterials and stem cells, and how to design a biomaterial to guide stem cell proliferation and development. These biomaterials can ultimately be used in regenerative medicine and stem cell therapy. Using a biodegradable novel polymer matrix, her research team has developed a cardiac patch that activates in vivo macrophage polarization, promoting cardiac regeneration. Utilizing human induced pluripotent stem cells, her research team has established physical approach for cultivating pluripotent stem cells and organoids resembling naïve tissues based on the physical properties of biomaterials. Additionally, Prof. Ma has played a key role in assisting clinical physicians in establishing clinical stem cell transformation processes compliant with GMP standards. As one of the main expert members, she successfully organized and participated in the development of steps and standards for Phase I, II, and III (multi-center) clinical stem cell cardiac transplantation and obtained approval from the ethics committee. She actively participated in supervising Phase III (PERFECT Trial) clinical stem cell heart transplantation therapy.
In terms of education, Prof. Ma serves as the spokesperson of the Helmholtz graduation school of macromolecular bioscience. She offers courses such as "Medical Biotechnology," "Stem Cell Biotechnology," "Stem Cell Biology and Clinical Translation," "Biological Macromolecules and Biology," and "Advances in Medicine" for undergraduate, master's, and doctoral students.
Funded grant:
• SFB Transregio 37 Biofunktionalisierte Mikro- und Nanofaserstrukturen (DFG)
• Nachwuchsgruppe Regenerative Medizin MV-2 (Junior research group of cardiovascular regeneration, Helmholtz-Gemeinschaft and Mecklenburg-Vorpommern)
• Intramural funding of Universität Rostock
• Forschungsfonds MV (Excellent Research Prize of Mecklenburg-Vorpommern): RNA modulation of stem cell fate
• BMBF BioChance plus stem cell funding: stem cell isolation with magnetic particle
• SFB 1112 Nanocarrier Z01: nanotoxicity and drug delivery (DFG)
• Intramural funding of FUB
• DAAD-China scientific exchange fund 2013-2015
• Helmholtz graduation school of macromolecular bioscience
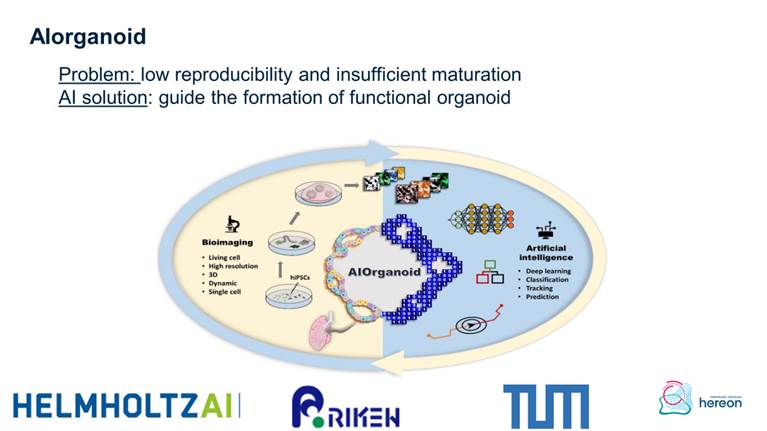
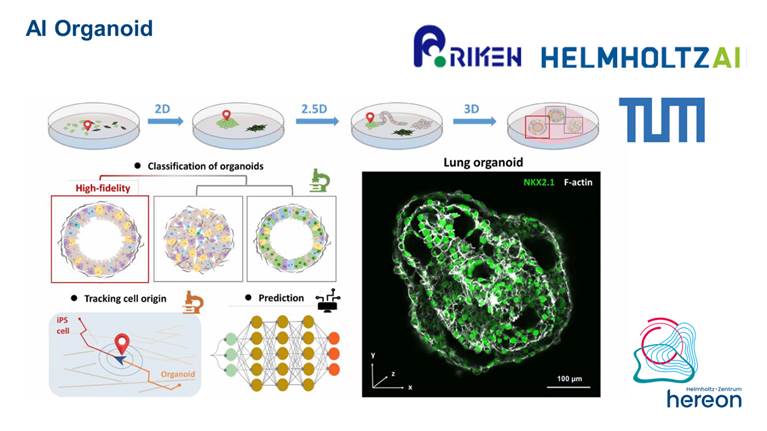
• Helmholtz artificial intelligent imaging project 2023-2025 AIorganoid
Publication: (197, selected publication)
• Xu, X, et al. Design criteria for geometrical cues reverting pluripotent stem cell state to naivety. Nature Materials (Accepted)
• Bhaskar T, et al. Advanced Functional Materials 33(46) September 2023, DOI: 10.1002/adfm.202304268
• Wing T, et al., Advanced Functional Materials. 27 May 2022, DOI: 10.1002/adfm.202110179
• Deng Z, et al. Proceedings of the National Academy of Sciences U S A 2020 Jan 28; 117(4):1895-1901.
• J. Balke et al. Small 2018, 14, e1800310.
• Cheng C, et al. Advanced Materials 2018. 30(5). 1705452
• Li M, et al. ACS Appl Mater Interfaces. 2017 11; 9(40):35411-35418.
• Vogt A, et al. J Control Release. 2016 Nov 28; 242:3-15.
• Wang W, et. al. J Control Release. 2016, 242, 71-79.
• Ren X, et al. Chemical Society Reviews 2015, 44 (15), 5680-5742
• T. Neffe, et al. Advanced Materials 2015, 27, 1738-1744. Cover article
• Xu X, et al. Advanced Healthcare Materials.2014 Dec;3(12):1991-2003
• Gaebel R, et al., Biomaterials 2011, 32, 9218-30.
Featured research:
Cardiac patch: In Vivo Performance of a Cell and Factor Free Multifunctional Fiber Mesh Modulating Postinfarct Myocardial Remodeling: in this research we design a polymeric cardiac patch to balance the immune response and regenerative process, alleviating the consequences of heart infarct. This bioinstructive mesh system, with tailored micromechanical properties and fiber arrangement, facilitates epicardial-derived cell adhesion and macrophage infiltration as well as cardiac tissue engraftment. In addition, it supports the M1/M2 transition of endogenous macrophages, and the release of proangiogenic factors, improved neovascularization, and contractile function. Neither the addition of cardiac extracellular matrix nor of induced cardiomyocyte progenitor cells further enhances the cardioprotective effect of the mesh. This cell- and factor-free system for treatment of postischemic myocardial dysfunction greatly simplifies manufacturing as well as regulatory processes and warrants further translational advancement toward a first-in-human clinical study.
https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202110179
2D stem cell niche:
Embryonic stem cells (ESCs) naturally interact with the basement membrane, which serves as their natural matrix/niche. It is very challenging to create basement membrane mimetics allow clonal derivation, survival, and long-term self-renewal of pluripotent stem cells under chemically defined and xeno-free conditions. In this research, the dynamic functions of laminin-111 (Lam-111) in ultrathin films at the air–water interface are explored. It is shown that the 2D confinement induces polymerization of laminin-111 and that expansion via adlayer formation occurs only with extended growth time. By assembling Lam-111 2D networks at the surface of Col-IV sheets, freestanding bilayers closely mimicking the basement membrane can be produced and supporting induced pluripotent stem cell attachment These fundamental studies highlight the importance of dynamic functions, encoded into the molecular structure of the building blocks, for the assembly, maintenance, and functioning of the complex material systems found in natural tissues and can provide cues for the molecular design of resilient technical systems.
https://onlinelibrary.wiley.com/doi/10.1002/adfm.202304268
Power of geometry
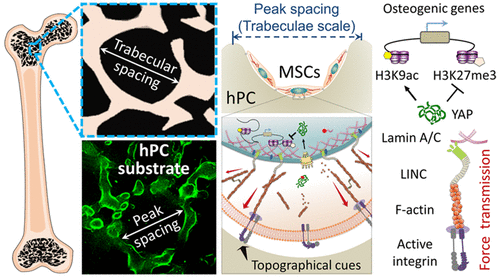
Drawing inspiration from the structure of trabecular bone, we engineered a 2.5D substrate characterized by an average peak spacing akin to the pore size of trabecular bone. This substrate demonstrated the potential to augment hBMSCs adhesion and promote cytoskeleton organization, thereby enhancing cytoskeletal tension. The increased cytoskeletal tension resulted in cell nuclear deformation and histone modification, subsequently influencing gene expression and guiding hBMSC differentiation lineage. Through mechanistic studies, we elucidated the roles of various components, including transmembrane, intracellular, and nuclear proteins, at the epigenetic level. Particularly, we underscored the significance of YAP and Lamin A/C in histone modification at differentiation gene loci during the mechanotransduction process. These findings underscore the potential of utilizing materials in conjunction with biochemical factors targeting YAP and Lamin A/C to optimize stem cell applications. This research bridges a crucial gap in our fundamental comprehension of cell-substrate interactions and underscores the importance of substrate topographical cues as a pivotal design element for regulating stem cells in bone repair and regeneration. Such insights are instrumental for advancing implant technology, offering a new direction for the design and development of implant devices.
https://pubs.acs.org/doi/10.1021/acsami.3c01481
AIorganoid